Introduction: Older patients with an age above 60 years with classical Hodgkin lymphoma (cHL) represent a proportion of 20% to 30% of all cHL. Older cHL patients are characterized by unfavorable prognostic factors with an aggressive disease, a poor tolerance to chemotherapy especially with bleomycin-induced lung toxicity resulting to a significant reduced survival as compared to younger patients.
PVAG regimen (prednisone, vinblastine, doxorubicin, gemcitabine) was developed by the German Hodgkin Study Group (GHSG) to improve results and reduce toxicities of ABVD regimen. In a prospective phase II study of 55 early unfavorable and advanced-stage elderly HL patients (median age, 68 years), 78% achieved complete response (CR) with a 3-year progression free survival (PFS) and overall survival (OS) rates of 58% and 66%, respectively (Böll et al, Blood 2011) with favorable toxicity profile. To the best of our knowledge, there is no report that described efficacy and toxicity of this protocol in real-life setting.
Methods: Between June 2011 and February 2020, 49 elderly patients with cHL received first-line chemotherapy with PVAG (Prednisone 40 mg/m2, Vinblastine 6 mg/m2, Doxorubicin 50 mg/m2, Gemcitabine 1000 mg/m2, or adapted-dose of PVAG) in 6 LYSA centers. All medical records were reviewed for clinical and biological characteristics, modality of treatment, responses and outcome. Comorbidities were evaluated according to the cumulative illness rating scale for geriatrics (CIRS-G) and treatment-related toxicity according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE).
Results: The median age of the 49 patients was 76 years (range, 61-87) with 44 patients ≥70 years old (69%) and 27 male (55%). Ann Arbor stages were as follows: II (n=16, 33%), III (n=12, 24%), IV (n=21, 43%). Altered performance status (PS 2-4) was presented in 35% of patients and B symptoms in 59%. IPS was ≥3 in 32 (65%) patients.
CIRS-G Grade 3 or 4 in two or more categories was observed in 11 patients (22%) and 22 patients (43%) had a cumulative CIRS-G score over 6.
Patients received a median of 6 cycles (range 1-8), 21 of them (43%) received adapted dose of PVAG. Seven patients (14%) received radiotherapy after respectively 3, 4, 6, or 8 cycles of PVAG.
At the end of PVAG regimen, 26 patients were in CR (53%), 4 PR (8%), 19 patients progressed (39%). For the 46 patients who were evaluated by PET-CT after chemotherapy, the CR and PR rates were 52% and 13% with 35% of patients with stable or progressive diseases.
For hematologic toxicity, 6 patients (12%) developed febrile neutropenia, 22 (45 %) had grade III-IV neutropenia; 8 (16 %) a grade 3-4 thrombopenia; 17 (35%) grade 3-4 anemia. Extra-hematologic toxicities were mild with three patients (6%) with grade 3-4 mucositis, 2 (4%) grade 3-4 nausea, 5 (10%) with grade 3-4 neuropathy, 3 (6%) acute heart toxicity.
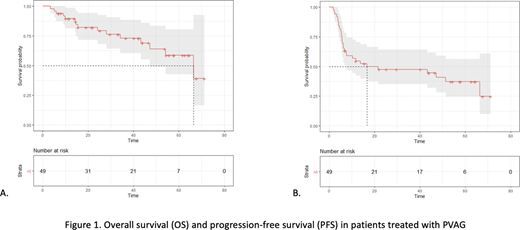
With a median follow up of 33,2 months (range, 14,3 -53,7), 26 (53%) patients progressed or relapsed. The median PFS was 21,6 months with a 3-year PFS rate of 48,6% (95%CI, 36,3-65,1). The median overall survival (OS) was 66,5 months with a 3-year OS rate of 73,7% (95%CI, 61,2-88,8). The cause of death was HL in 8 patients (16%), infection in 2 (4%); one toxic death occurred (sepsis after first cycle of PVAG).
In univariate analysis, PFS (HR: 2,36, 95CI, 1,01-5,48, P=0.0,046) and OS (HR: 4,23, 95%CI, 1,15-15,6, P=0.03) were adversely affected by high number of medications (>3). OS was adversely affected by grade 3-4 CIRS-G in ≥2 categories (HR: 3,63, 95%CI, 1,23-10,71, P=0.019). Age, IPS, presence of B symptoms, lymphopenia, anemia, low albumin level, CIRS-G>6 did not affect outcome.
Conclusions:Our real-life evaluation of PVAG regimen showed that patients were older than those included in the pivotal clinical trial and 58% of patients received adapted-dose of chemotherapy. We confirmed the favorable safety profile of this protocol. Using TEP-scan evaluation, the CR rate was 52%. Survival analyses supported initial results obtained in clinical trial. Combinations with immunotherapies with clinical activity in cHL should be evaluated to improve results of this regimen.
Brice:Takeda: Consultancy; Roche: Consultancy. Salles:Epizyme: Honoraria, Other: consultancy or advisory role; Kite, a Gilead Company: Honoraria, Other: consultancy or advisory role ; Janssen: Honoraria, Other: consultancy or advisory role; BMS/Celgene: Honoraria, Other: consultancy or advisory role; Takeda: Honoraria; Karyopharm: Honoraria; Genmab: Honoraria, Other; Debiopharm: Consultancy, Honoraria, Other: consultancy or advisory role; MorphoSys: Honoraria, Other: consultancy or advisory role; Novartis: Honoraria, Other: consultancy or advisory role; Roche: Honoraria, Other: consultancy or advisory role; Abbvie: Other: consultancy or advisory role; Autolos: Other: consultancy or advisory role. Deau Fischer:Takeda: Consultancy; Roche: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal